Pharmacogenomics
Understand how variations in the human genome affect our response to medications
Using pharmacogenomics to maximize the benefits of medications and reduce health care costs
Benefits of Pharmacogenomics
Pharmacogenomics (PGx) is the study of how variations in the human genome dictate a person’s response to medications. In one study, more than 99% of people assessed had a genotype associated with a higher risk to at least one medication.1 Findings from pharmacogenomics research can lead to better future outcomes for both individuals and healthcare providers through improved medication safety and efficacy and lowered medical costs.
Leveraging pharmacogenomics technologies will ultimately enable healthcare providers to:
- Maximize the intended use of a medication or treatment
- Reduce adverse drug reactions
- Speed time to achieving the therapeutic benefit of a drug
- Decrease the chance of side effects or dependency
Decrease the cost of healthcare expenditures by:
- Using genomics to identify the most appropriate and affordable drug the first time
- Reducing adverse drug reactions early in treatment, thus
- Reducing hospital length of stays
- Reducing hospital readmissions
- Reducing ER visits
Cost-Effectiveness of Pharmacogenomic (PGx) Testing
Health economics and research findings support PGx testing. See the data supporting the value of PGx for institutions, physicians, and patients, and learn how PGx testing improves care.
View Infographic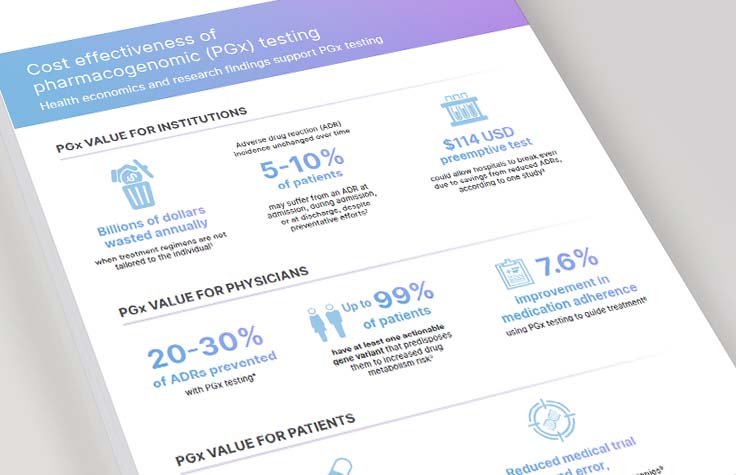
Technology Comparison
Advantages and Disadvantages of Common PGx Technologies2
| Technology | Advantages | Disadvantages |
|---|---|---|
| Sanger Sequencing |
|
|
| Real-Time PCR |
|
|
| Microarrays |
|
|
| Next-Generation Sequencing (NGS): Exome and Whole-Genome Sequencing |
|
|
Microarrays for Pharmacogenomics Research
Microarrays have become an important technology in precision medicine, enabling clinical researchers to make significant advancements in the area of PGx research. The Infinium Global Diversity Array with Enhanced PGx Content provides coverage of high-priority PGx genes, representing a major step forward:
- Genome-wide coverage of >44k PGx biomarkers
- Automated, scalable workflow with a 3-day turnaround time
- >6k variants from PGx databases, including PharmGKB, CPIC, PharmVar, and ClinVar
- ~500 CPIC variants available for interrogation, with >300 of them priority level A or B based on the strength of supporting clinical evidence3
- High-impact, historically hard-to-discern PGx genes like CYP2D6, CYP2B6 and TPMT are now accessible due to workflow improvements that enable pseudogene disambiguation
Infinium Global Diversity Array with Enhanced PGx Content
Introducing the most comprehensive genotyping microarray on the market for pharmacogenomics research with >1.9M markers, access to high-impact PGx genes, and optional reporting software.
This trusted Infinium assay has been run on millions of samples, including use in the All of Us Research Program. It provides a cost-effective, end-to-end solution with star allele calling and metabolizer status reporting, allowing for consolidation of multiple assays onto a single chip. View Array
Featured Pharmacogenomics Research Workflow
This rapid, three-day workflow allows users to gather and report data quickly and run up to 1728 samples per week using a single iScan System. Targeted gene amplification runs concurrently with whole-genome amplification during day 1 of the 3-day protocol. The workflow is highly scalable and can be automated with robotic liquid handlers.
Process
Scan
Track, Analyze & Report
Featured Bundles
Contact us to discuss your PGx needs.
Featured Webinars
Personalized Medicine Based on Common and Rare Genetic Variants
Dr. Lili Milani and her team at the Estonian Biobank have been using OMICS profiling data (including WGS, WES, and genotyping) from biobank participants to identify rare mutations, develop polygenic risk scores, and conduct PGx research.
The team is looking for genetic variants associated with adverse reactions to specific medications and studying how to translate existing genomic data into meaningful guidelines.
View WebinarImplementing PGx in Estonian Healthcare
Dr. Tonu Esko describes the Estonian Biobank initiative and its efforts to implement pharmacogenomics at the national healthcare level. He talks about two ongoing pilot prevention programs for cardiovascular diseases and breast cancer.
His presentation covers collection of polygenic risk score research data from biobank participants, ways of integrating PGx screening programs into the clinical setting, and media strategies to sensitize public opinion on the benefits of PGx initiatives.
View WebinarIdentify Novel Pharmacogenomic Biomarkers
Whole-genome sequencing with NGS technology provides a high-resolution, base-by-base view of the entire genome, ideal for discovery applications such as novel PGx biomarker identification.
Learn More About WGSScientists Discuss Pharmacogenomics Applications
Bringing Meaning to Genetic and PGx Information
MyDNA co-founder Allan Sheffield discusses development of a pharmacogenomics service and meaningful genetic test reports.
Genetic and PGx Data Matchmaking for Researchers
A biobank project helps scientists connect with those who wish to share their genetic data for research and have rare genetic variants associated with outcomes such as metabolizing certain medicines differently.
Scaling an Efficient Genotyping Facility
The cofounder of a company focused on PGx, nutrigenomics, and chronic diseases discusses switching from real-time PCR to high-throughput microarray and next-generation sequencing technologies.
Featured Podcasts
The Impact of PGx on Precision Medicine
Dr. Howard McLeod, PharmD discusses how genes affect drug metabolism, how pharmacogenomics is turning "fishing expeditions" into precision care, and what needs to be done to integrate PGx applications into routine care.
Listen to PodcastBarriers and Opportunities to PGx Implementation
Dr. Ronald Leopold discusses implementation of PGx screening in the healthcare industry. The conversation explores barriers to PGx program adoption, the future of precision medicine, and a paradigm shift away from reimbursement to value-based healthcare.
Listen to PodcastRelated Solutions
Oncology
Illumina NGS and microarray technologies for cancer research are helping drive the revolution in cancer genomics.
Complex Disease Genomics
Comprehensive array and next-generation sequencing solutions to accelerate research of various genetic complex diseases.
Polygenic Risk Scores
Polygenic risk scores represent the total number of genetic variants an individual has that increase their risk of developing a particular disease.
Drug Discovery and Development
NGS can help pharmaceutical scientists identify potential drug targets, investigate disease-associated genetic variants, and develop targeted therapies.
Microbiome Analysis
Analyze the human microbiome with experimental techniques such as shotgun metagenomics, 16S rRNA metagenomics, and metatranscriptomics.
Neurogenomics
Genomic neuroscience research with next-generation sequencing and microarray technologies is advancing our understanding of neurological diseases and the nervous system.
Featured Publications
Clinical implementation of pharmacogenomics via a health system-wide research biobank: the University of Colorado experience
Translating pharmacogenomics into clinical decisions: do not let the perfect be the enemy of the good
Translating genotype data of 44,000 biobank participants into clinical pharmacogenetic recommendations: challenges and solutions
Economic burden of adverse drug reactions and potential for pharmacogenomic testing in Singaporean adults
References
- Reisberg S et al. Translating genotype data of 44,000 biobank participants into clinical pharmacogenetic recommendations: challenges and solutions. Genet Med. 2019 Jun;21(6):1345-1354.
- Catriona Hippman and Corey Nislow. Pharmacogenomic Testing: Clinical Evidence and Implementation Challenges. J Pers Med. 2019 Sep; 9(3): 40.
- The FDA has evaluated a large number of pharmacogenetic associations and documented those in which sufficient evidence suggests a sub-population of people would exhibit altered drug metabolism. See Table of Pharmacogenetic Associations




